Osmosis
Consider two water solutions, one of which is 0.9% NaCl and one of which is 2% NaCl, separated by a semipermeable membrane. The semipermeable membrane allows water molecules to pass back and forth from one solution to another, but it blocks the sodium and chloride ions from passing through. More water will move across the membrane from the more dilute 0.9% NaCl solution that has a higher concentration of water than from the more concentrated 2% NaCl solution that has a lower concentration of water, so there will be a net shift of water from the side of the tube with the dilute solution to the side of tube with the more concentrated solution. This process will continue until an equilibrium is reached where the rates of movement of water back and forth through the membrane are equal.
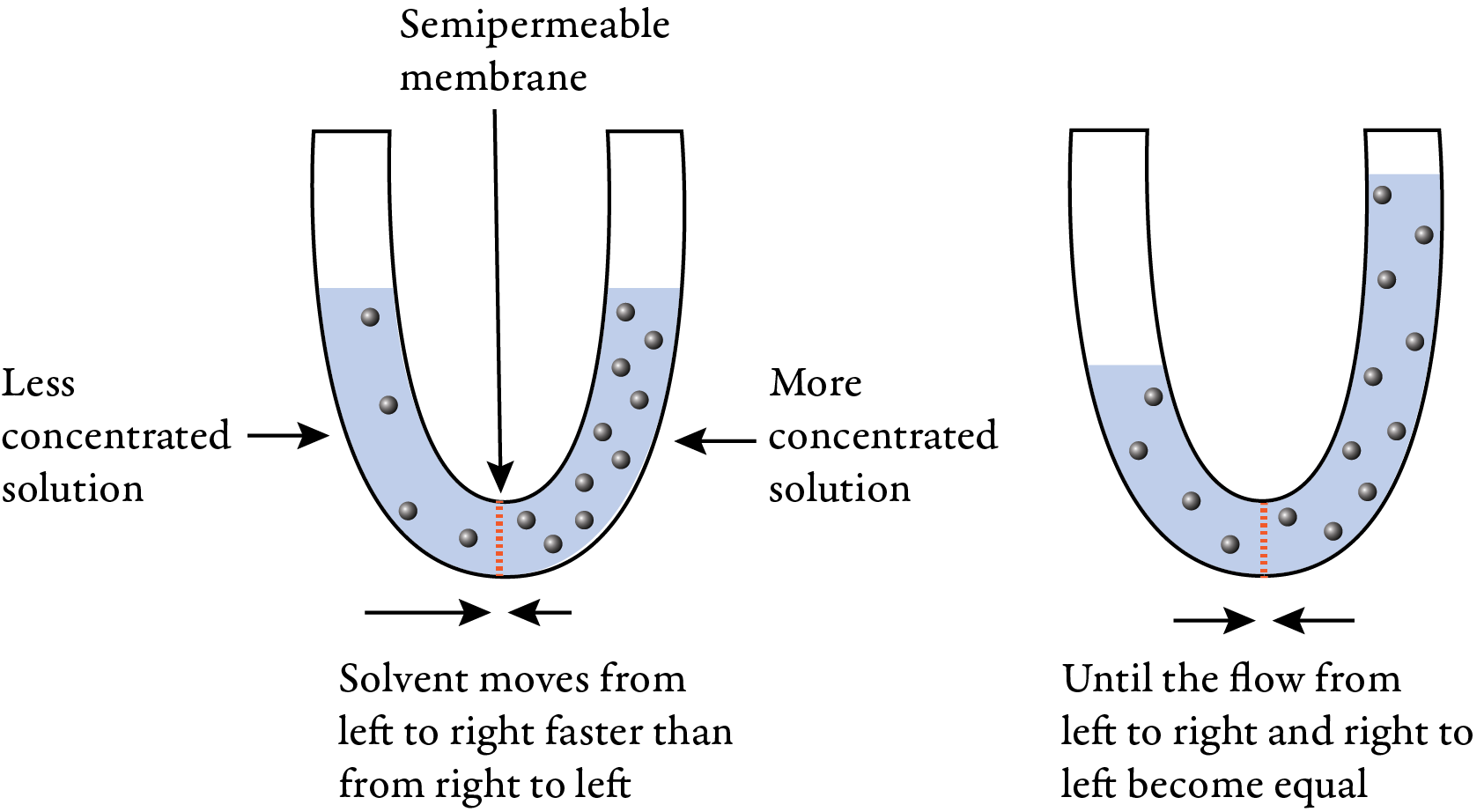
This process is called osmosis, which is the spontaneous net movement or diffusion of solvent through a semipermeable membrane from pure solvent or a solution that is less concentrated in solute (more concentrated in solvent) to a solution that is more concentrated in solute (less concentrated in solvent). Osmosis tends toward equalizing the concentrations on each side of the membrane.
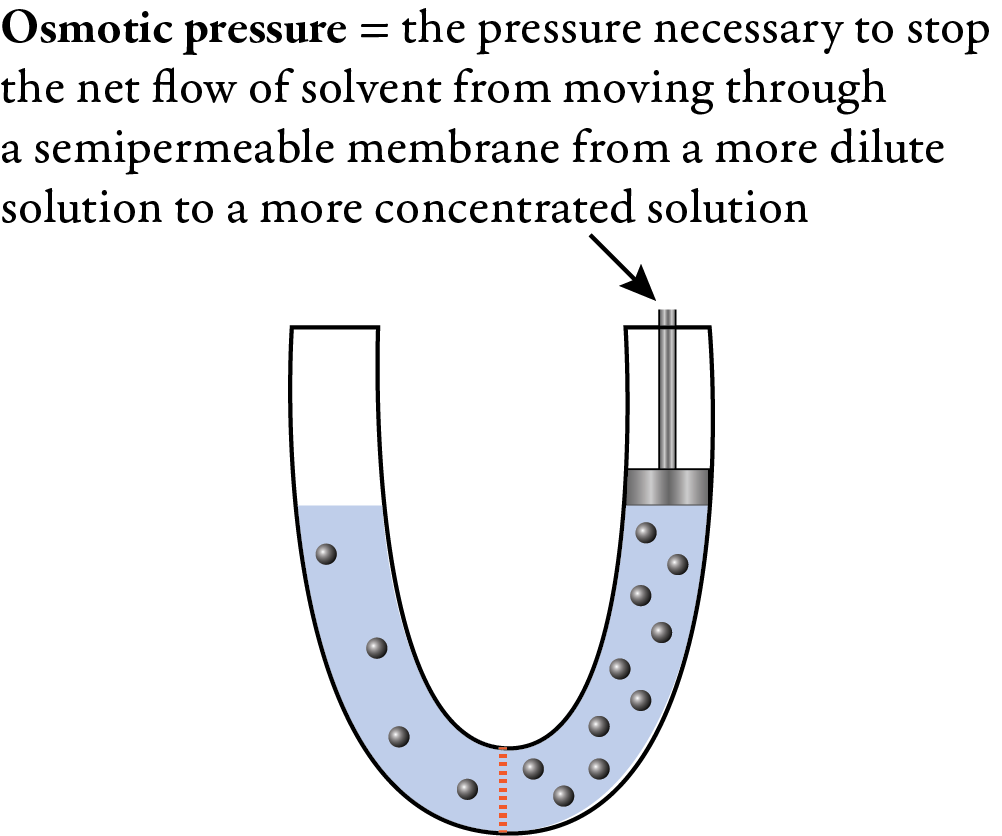
You can think of this net movement of solvent through the membrane as generating a pressure, so it must be possible to stop the net flow of solvent through the membrane by applying an external pressure to the more concentrated solution. The external pressure necessary to stop the net flow of solvent through the membrane is called the osmotic pressure. Because the osmotic pressure is dependent on the relative concentrations of the two solutions and not on the nature of the chemical components in the solutions, it is a colligative property.
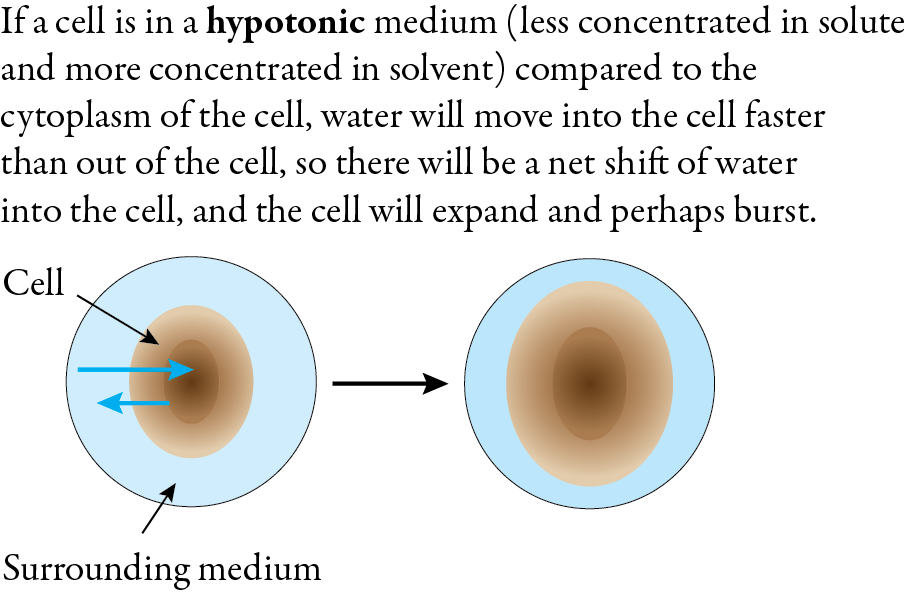
Osmosis is the primary mechanism for the movement of water in and out of cells in biological systems. When a cell is submerged in a hypotonic solution, which has a lower concentration of solute and a higher concentration of water, the net flow of water is into the cell. Plants get water from their roots via osmosis, causing a buildup of pressure in the plant cells (called turgor pressure). This pressure allows herbaceous plants to stand upright. If a red blood cell is submerged in a hypotonic solution, and if enough water moves into the cell to cause it to expand and burst, it will release hemoglobin. This process is called hemolosis.
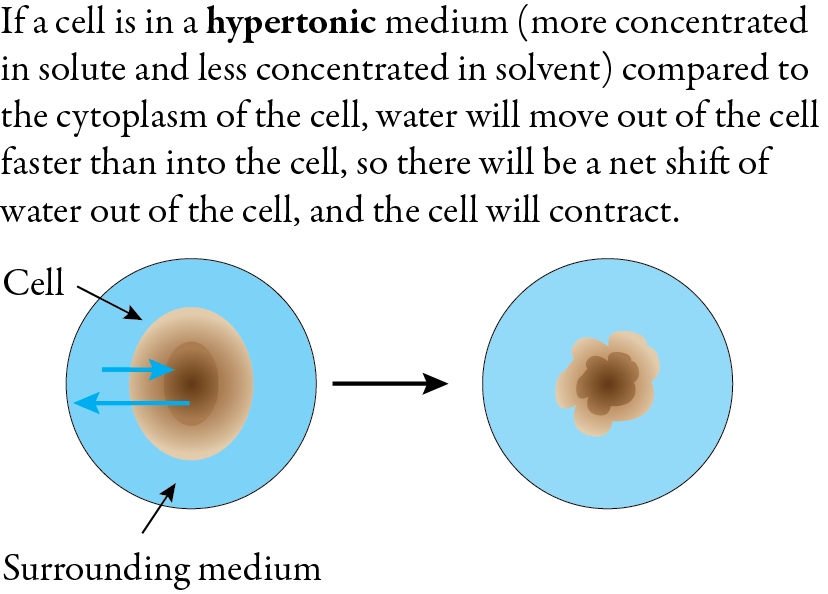
When a cell is submerged in a hypertonic solution, which has a higher concentration of solute and a lower concentration of water, the net flow of water is out of the cell. This causes the cell to shrink and become crenated (scalloped or notched). This process is called crenation.
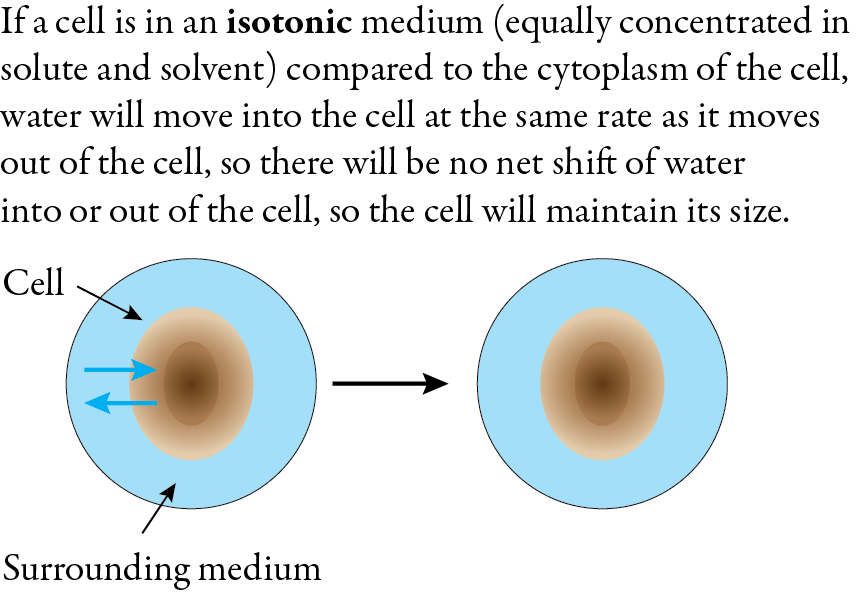
When a cell is submerged in an isotonic solution, which has the same concentration of solute and water, there is no net flow of water into or out of the cell. The cell maintains its size and shape.
Water can be separated from seawater by reverse osmosis. In this process, the seawater is separated from more pure water by a semipermeable membrane, and an external pressure on the seawater side that is greater than the osmotic pressure of the seawater (about 27 atm) pushes the water through the membrane from the seawater side to the more pure water side.
