Converting Names to
Formulas
Before you can write a
chemical formula from the name of a compound, you need to recognize what type of
compound the name represents. For binary ionic compounds, the first part of the
name is the name of a metallic cation. This may include a Roman numeral in
parentheses. The anion name starts with the root of the name of a nonmetal and
ends with ‑ide.
(name of metal)(maybe Roman numeral) (root of nonmetal)ide
For example, aluminum fluoride (used in the production of aluminum) and tin(II) chloride (used in galvanizing
tin) are binary ionic compounds.
You can identify other names
as representing ionic compounds by recognizing that they contain the names of
common polyatomic ions. For example, ammonium chloride and iron(III) hydroxide
are both ionic compounds. Many of the polyatomic ions that you will be expected
to recognize end in ‑ate, so this ending tells you that the name
represents an ionic compound. Copper(II) sulfate is an ionic compound.
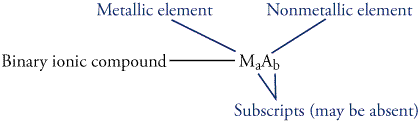
Follow these steps to write formulas for ionic compounds.
Step 1: Write the formula, including the charge, for the cation.
Step
2: Write the formula, including the charge, for the anion.
Step 3: Write subscripts for each formula so as to yield an
uncharged compound. ( Use the lowest whole number
ratio for the subscripts. If the subscript
for a polyatomic ion is higher than one, place the formula for the polyatomic
ion in parentheses and put the subscript outside the parentheses.)
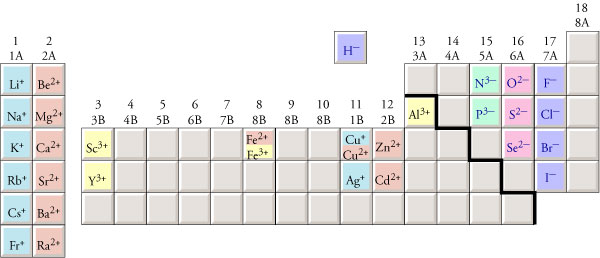
The image below shows
the formulas for monatomic
ions for which you should know the charges.
You should know the names and formulas for the following
polyatomic ions.
hydroxide ion, OH-
nitrate ion, NO3-
acetate ion, C2H3O2-
carbonate ion, CO32-
sulfate ion, SO42-
phosphate ion, PO43-
Some polyatomic anions are
formed by the attachment of one or more hydrogen atoms. In fact, it is common
for hydrogen atoms to be transferred from one ion or molecule to another ion or
molecule. When this happens, the hydrogen atom is usually transferred without
its electron, as H+. If an anion has a charge of -2 or -3, it can
gain one or two H+ ions and still retain a negative charge. For
example, carbonate, CO32-, can gain an H+ ion
to form HCO3-, which is found in baking soda. The sulfide
ion, S2-, can gain one H+ ion to form HS-.
Phosphate, PO43-, can gain one H+ ion and form
HPO42-, or it can gain two H+ ions to form H2PO4-.
These polyatomic ions are named with the word hydrogen in front of the
name of the anion if there is one H+ ion attached and dihydrogen
in front of the name of the anion if two H+ ions are attached.
HCO3- is hydrogen carbonate ion.
HS- is hydrogen sulfide ion.
HPO42- is hydrogen phosphate ion.
H2PO4- is dihydrogen
phosphate ion.
See the following links to get more information on writing formulas for\cations and anions.
Cation Names and Formulas
Anion Names and
Formulas